Iris yellow spot orthotospovirus pathosystem, virus host and vector (Thrips tabaci)
By
Norma Ávila Alistac*,
Erika J. Zamora Macorra,
Héctor Lozoya Saldaña
* Corresponding Author. Email: / Institution: Universidad Autónoma Metropolitana
Received: 30/October/2023 – Published: 10/March/2024 – DOI: https://doi.org/10.18781/R.MEX.FIT.2310-8
Abstract Iris yellow spot Orthotospovirus (IYSV) causes serious problems in the onion (Allium cepa) crop and is widely distributed in the producing areas of the country. In Mexico it was reported in 2010 as “yellow spot” on onion and other members of the genus Allium. Its main vector is Thrips tabaci, which causes direct damage by feeding and by being a vector of other viruses such as Tomato spotted wilt Orthotospovirus and Impatiens necrotic spot Orthotospovirus. Knowledge of the pathosystem of IYSV - Thrips tabaci - Allium cepa - weeds can contribute to an integrated management and awareness of pesticide use. The versatility of IYSV to infect more than 60 plant species (>20 families), most of which are present in Mexico, coupled with the wide host range of the vector, makes the interaction complex and leads to a better understanding of the diversity of alternate hosts of the vector and/or IYSV. At present, information on weed hosts of IYSV and the vector is limited, but their knowledge will provide a greater understanding of the disease. It is important to have a comprehensive knowledge of the virus, main host, alternate hosts, and vector in the country, in order to channel future research to counteract this problem and minimize losses caused by IYSV in the onion crop mainly
Keywords:
IYSV, Allium, weeds, host, orthotospovirus
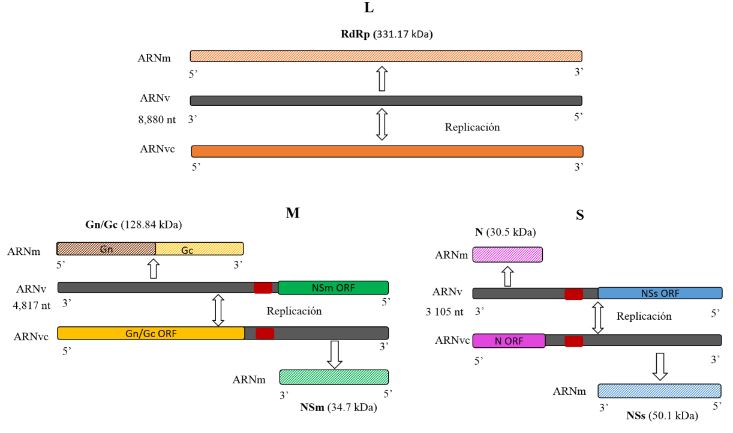
Figure 1. Representation of the segmented organization of the Iris yellow spot Orthotospovirus RNA genome (L, M and S). Modified from Bag et al. (2015)
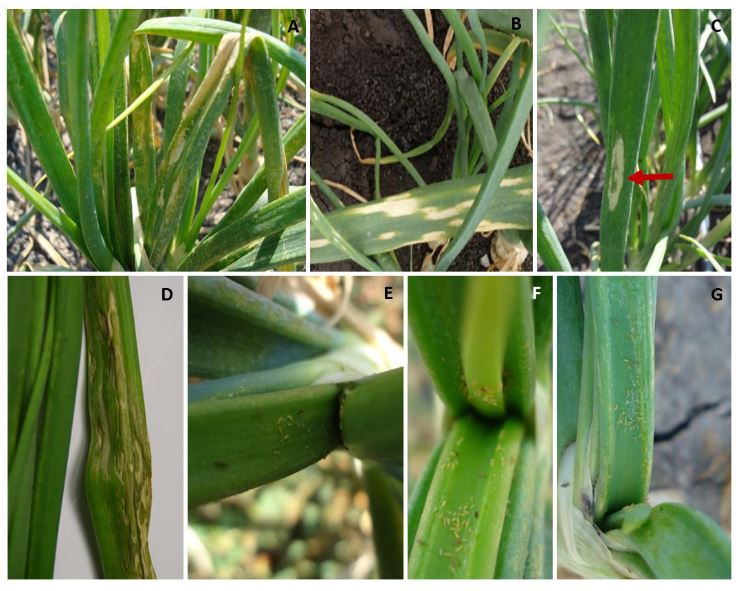
Figure 2. A and B) symptoms associated with the Iris yellow spot Orthotospovirus infection, consisting of pale straw- colored chlorotic lesions with an elongated shape. C and D) pale straw-colored chlorotic lesions with a green island in the center of the lesion E-G) presence of large T. tabaci populations (immature and adult) in the onion crop.

Figure 3. Weeds interacting inside and outside of the onion (Allium cepa) crop. A) Parthenium weed (Parthenium hysterophorus); B) Purslane (Portulaca oleracea); C) Pineland threesed mercury (Acalypha ostryifolia); D) Mexican sunflower (Tithonia tubiformis) on the edge of an onion crop.
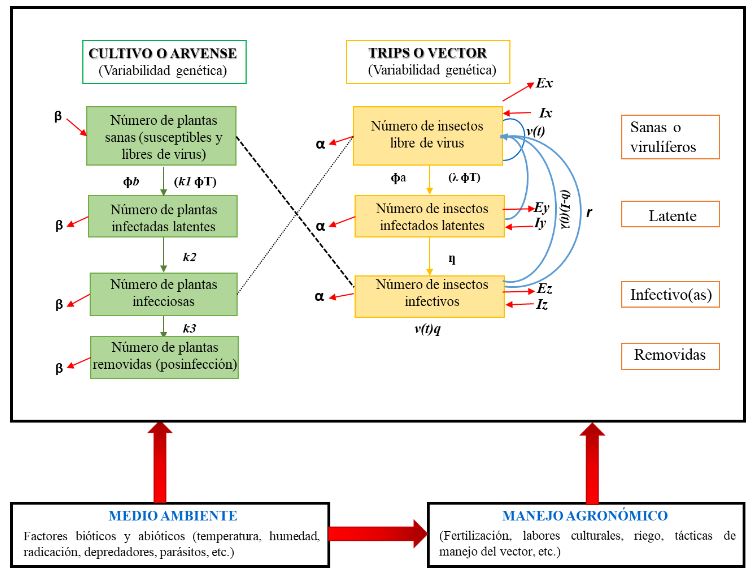
Figure 4. Epidemiological system of a viral pathosystem focused on Iris yellow spot Orthotospovirus (based on and modified from Madden et al., 2000). Notes used: v(t)= Total fertility rate of the insect population per day within the crop. Ґ= Virus incubation time within the vector. q= Probability that the offspring of the vector will be viruliferous. β= Plant mortality and recovery rate (replanting). α= Insect mortality and birth rate. ɸb= (Plants visited by insects per day) (Probability that the viruliferous insect inoculates the virus in a plant per visit). 1/k1= Time taken for the vector to inoculate the plant. 1/k2= Virus latency period in the plant. 1/k3= Infectious period of the virus in the plant. ɸT= (Plants visited by insects per day) (Time taken for the insect to sample the plant in one visit). Ex= Rate of emigration of virus-free insects. Ix= Rate of emigration of virus-free insects. Ey= Rate of emigration of latent insects. Ez= Rate of emigration of infective insects. Iy= Rate of emigration of latent insects. Iz= Rate of immigration of infective insects. 1/λ = Vector acquisition time. ɸa= (Plants visited by insects per day) (Probability that an insect will acquire the virus with a single visit to the infected plant). 1/ƞ= Time taken for the virus to transition from latent to infective within the vector (latency period). For further information, refer to Madden et al. (2000)
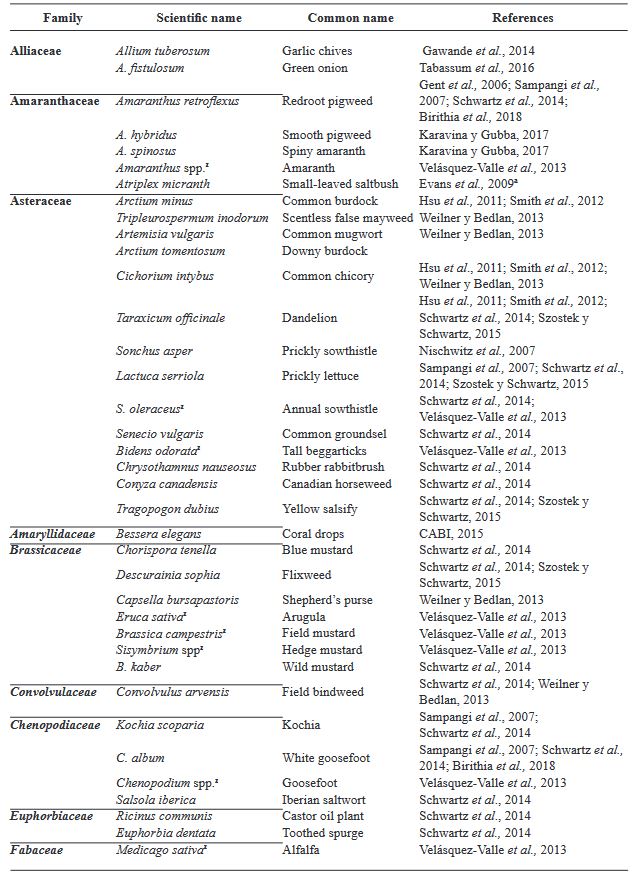
Table 1. Range of Iris yellow spot Orthotospovirus hosts

